Circular eGene™ construct amplification for large-volume applications
After screening your Circular eGene™ constructs on the eProtein Discovery™ system, you may require more material than was originally supplied, particularly for large-scale cell-free protein synthesis (CFPS). This protocol outlines a standard approach to generating larger quantities through E. coli transformation, culture growth, and plasmid purification using a Midiprep kit. Following these guidelines will help produce more Circular eGene™ material, at the quality required, for downstream applications.
Reagents and materials required
Table 1. Materials list.| Item |
|---|
| 1–10 ng of circular eGene™ construct |
| DH5-α competent cells (e.g. NEB® 5-alpha) |
| LB-Kanamycin plates (50 µg/mL Kanamycin) |
| Kanamycin stock solution (50 mg/mL) |
| LB media (100 mL) |
| PureLink™ HiPure Plasmid Midiprep Kit (Catalog number- K210004, ThermoScientific) |
| Isopropanol |
| 70% ethanol prepared in nuclease free water |
| Nuclease free water |
| Sterile bacterial culture tubes (14 mL size) |
| Sterile Baffled bacterial culture flask with filter cap (250 mL) |
Equipment required
Table 1. Equipment list.| Item |
|---|
| +37 °C incubator and shaker |
| Microbiological safety cabinet |
| Cooling centrifuge with rotor for 15 and 50 mL centrifuge tubes and speed up to 20,000 g |
Protocol
E. coli transformation and culture preparation
- Place a vial of competent E. coli DH5-α cells (e.g. NEB® 5-alpha) on ice until fully thawed (~5 min).
- Add 1–10 ng of vendor-supplied circular eGene™ plasmid (not linearized) directly to the thawed cells.
- Follow the manufacturer’s instructions for transforming the cells.
- Spread the transformation mixture onto LB agar plates containing 50 µg/mL kanamycin.
- Incubate at +37 °C for 14–16 hours.
- Pick a single, well-isolated colony and inoculate 2 mL LB broth containing 50 μg/mL kanamycin in a sterile 14 mL culture tube.
- Incubate overnight at +37°C with shaking at 250 rpm.
- Transfer 700 μL of the overnight culture into 70 mL LB broth containing 50 μg/mL kanamycin in a sterile 250 mL baffled flask.
- Incubate overnight at +37°C with shaking at 250 rpm.
- Transfer 50 mL of the culture into a 50 mL centrifuge tube.
- Centrifuge at 4000 ×g for 10 min to pellet the cells.
- Carefully discard the supernatant and invert the tube on absorbent paper to remove excess liquid.
- Continue immediately with plasmid purification or store the cell pellet at –80°C until needed.
Plasmid DNA purification
Kit recommendation: Use a plasmid Midiprep kit that produces nuclease- and endotoxin-free plasmid DNA (pDNA). We have tested and recommend: PureLink™ HiPure Plasmid Midiprep Kit (Thermo Scientific, Cat. No. K210004).
- Follow the manufacturer’s instructions for plasmid purification.
- Elute the DNA in 5 mL of the kit-supplied elution buffer.
DNA from the HiPure kit will be highly diluted. A precipitation step is required to concentrate the plasmid into nuclease-free water.
Plasmid DNA precipitation and quantification
- Add 3.5 mL of isopropanol to the eluted DNA. Mix well and incubate for 5 min at room temperature.
- Centrifuge at 16,000 ×g for 30 min at +4°C.
- A white DNA pellet should be visible at the bottom of the tube. Carefully remove the supernatant without disturbing the pellet.
- Add 3 mL of freshly prepared 70% ethanol (in nuclease-free water). Invert the tube 4–5 times to wash the pellet.
- Centrifuge at 16,000 ×g for 20 min at +4°C.
- Carefully remove the supernatant. Ensure the pellet remains intact.
- Briefly centrifuge again for 1 min and remove any remaining ethanol.
Important: Residual ethanol will make it difficult to fully dissolve the DNA. - Remove the cap and air-dry the pellet for 2–5 min until no liquid remains.
- Add 50 µL nuclease-free water to the pellet.
- Pipette up and down 10 times using a 100 μL pipette set to 50 μL.
- Cap the tube, spin briefly, and transfer the DNA to a sterile, low-bind 1.5 mL microcentrifuge tube.
- Measure the DNA concentration (260 nm). Expected yield: ~1000 ng/µL.
DNA normalisation
- Calculate molarity using the following formula to convert plasmid DNA concentration to molarity:
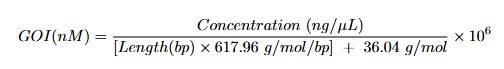
- Dilute the plasmid DNA to 100 nM using nuclease-free water.
Adaptation to the Circular eGene™ Workflow
Follow the instructions in the Circular eGene™ Preparation Kit Guide to prepare constructs for Cell-free Scale-up, or proceed with E. coli-based Scale-up.
Disclaimers: PureLink™ is a registered trademark of Thermo Fisher Scientific. NEB is a registered trademark of New England Biolabs, Inc. Product codes and catalog numbers are provided for reference only and may vary between regions or distributors. Please check with your local supplier for availability and compatibility.Nuclera Technical Support:
UK Phone +44 1223 942 761
US Phone: +1 508-306-1297
Email: techsupport@nuclera.com
Offices:
Nuclera UK (HQ):
One Vision Park, Station Road, Cambridge, CB24 9NP, UK
Nuclera USA: 1000 Technology Park Drive, Suite B, Billerica MA 01821, USA www.nuclera.com
Copyright © 2025 Nuclera Ltd. All trademarks are the property of Nuclera, Ltd. Visit nuclera.com/legal for more info.